Endoscopic Stricture Management Device Market Research Report
Endoscopic Stricture Management Device Market - Global Industry Size & Growth Analysis 2020-2033
Global Endoscopic Stricture Management Device Market is segmented by Application (Healthcare, Hospitals, Clinics, Research, Medical Devices), Type (Stent-based Devices, Balloon Dilators, Endoscopic Laser Devices, Electrocautery Devices, Endoscopic Forceps), and Geography (North America, LATAM, West Europe, Central & Eastern Europe, Northern Europe, Southern Europe, East Asia, Southeast Asia, South Asia, Central Asia, Oceania, MEA)
Pricing
Industry Overview
Global Endoscopic Stricture Management Device Market Size, Forecast, Segment Analysis, By Type Stent-based Devices, Balloon Dilators, Endoscopic Laser Devices, Electrocautery Devices, Endoscopic Forceps By Application Healthcare, Hospitals, Clinics, Research, Medical Devices, By Region North America, LATAM, West Europe, Central & Eastern Europe, Northern Europe, Southern Europe, East Asia, Southeast Asia, South Asia, Central Asia, Oceania, MEA (2025 to 2033)
The endoscopic stricture management device market includes medical devices used for diagnosing and treating strictures in the gastrointestinal tract. These devices help in minimally invasive procedures such as dilation, stenting, and tissue removal. The market is expanding due to the rise in gastrointestinal diseases and demand for less invasive treatment options.
The research study Endoscopic Stricture Management Device Market provides readers with details on strategic planning and tactical business decisions that influence and stabilize growth prognosis in Endoscopic Stricture Management Device Market. A few disruptive trends, however, will have opposing and strong influences on the development of the Global Biometric Lockers market and the distribution across players. To provide further guidance on why specific trends in Endoscopic Stricture Management Device market would have a high impact and precisely why these trends can be factored into the market trajectory and the strategic planning of industry players.
Market Dynamics Highlighted
Market Driver
The Endoscopic Stricture Management Device Market is experiencing significant growth due to various factors.
- • Rising prevalence of gastrointestinal diseases
- • increasing demand for minimally invasive treatments
- • growth in endoscopic procedures
- • rise in elderly population
- • increasing healthcare expenditure.
Market Trend
The Endoscopic Stricture Management Device market is growing rapidly due to various factors.
- • Growth in demand for minimally invasive procedures
- • increasing adoption of endoscopic treatments for strictures
- • rise in demand for stent-based solutions
- • technological advancements in endoscopic devices
- • growth in outpatient treatment options.
Opportunity
The Endoscopic Stricture Management Device has several opportunities, particularly in developing countries where industrialization is growing.
Challenge
The market for fluid power systems faces several obstacles despite its promising growth possibilities.
Endoscopic Stricture Management DeviceMarket Segment Highlighted
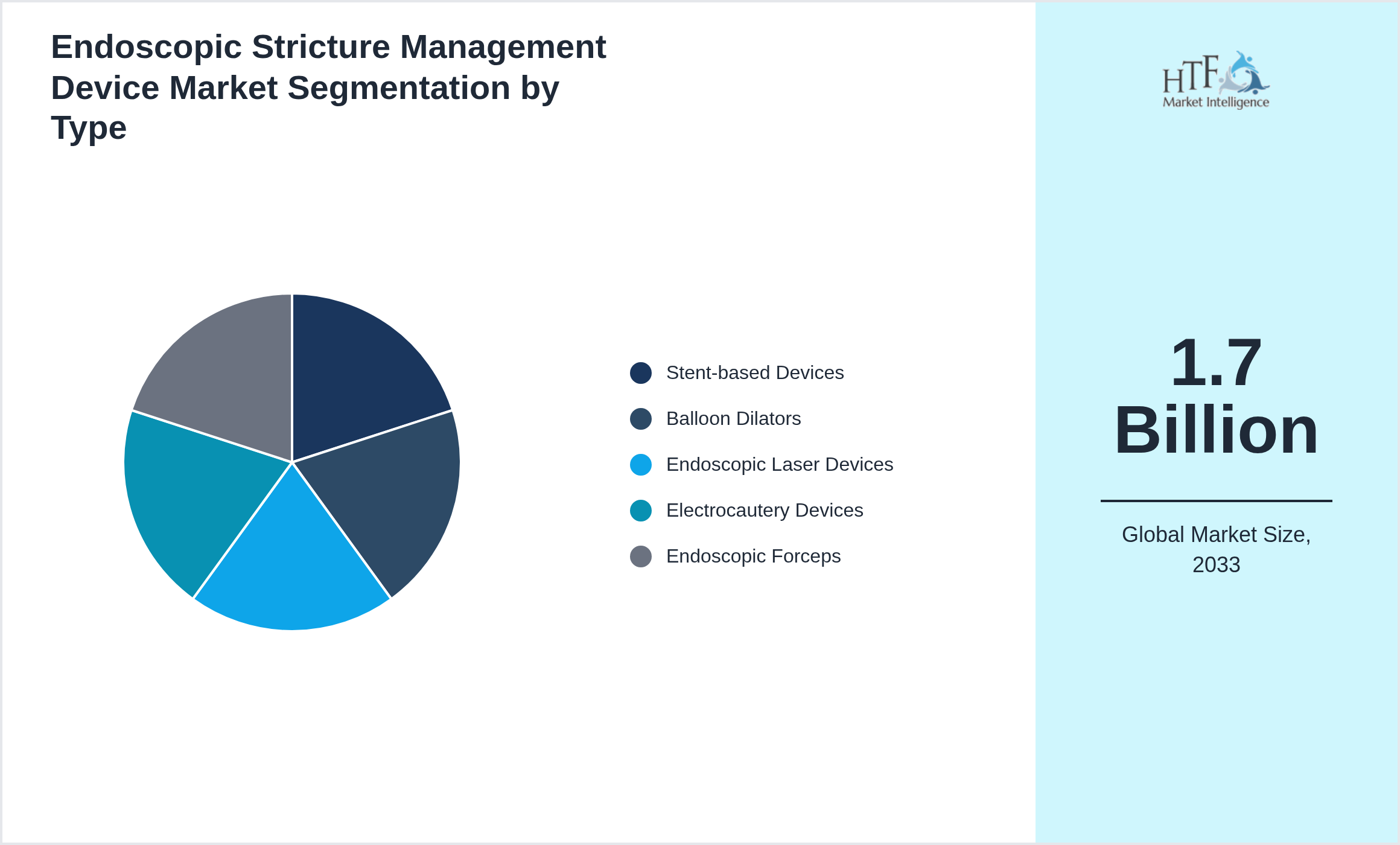
Segmentation by Type
- • Stent-based Devices
- • Balloon Dilators
- • Endoscopic Laser Devices
- • Electrocautery Devices
- • Endoscopic Forceps
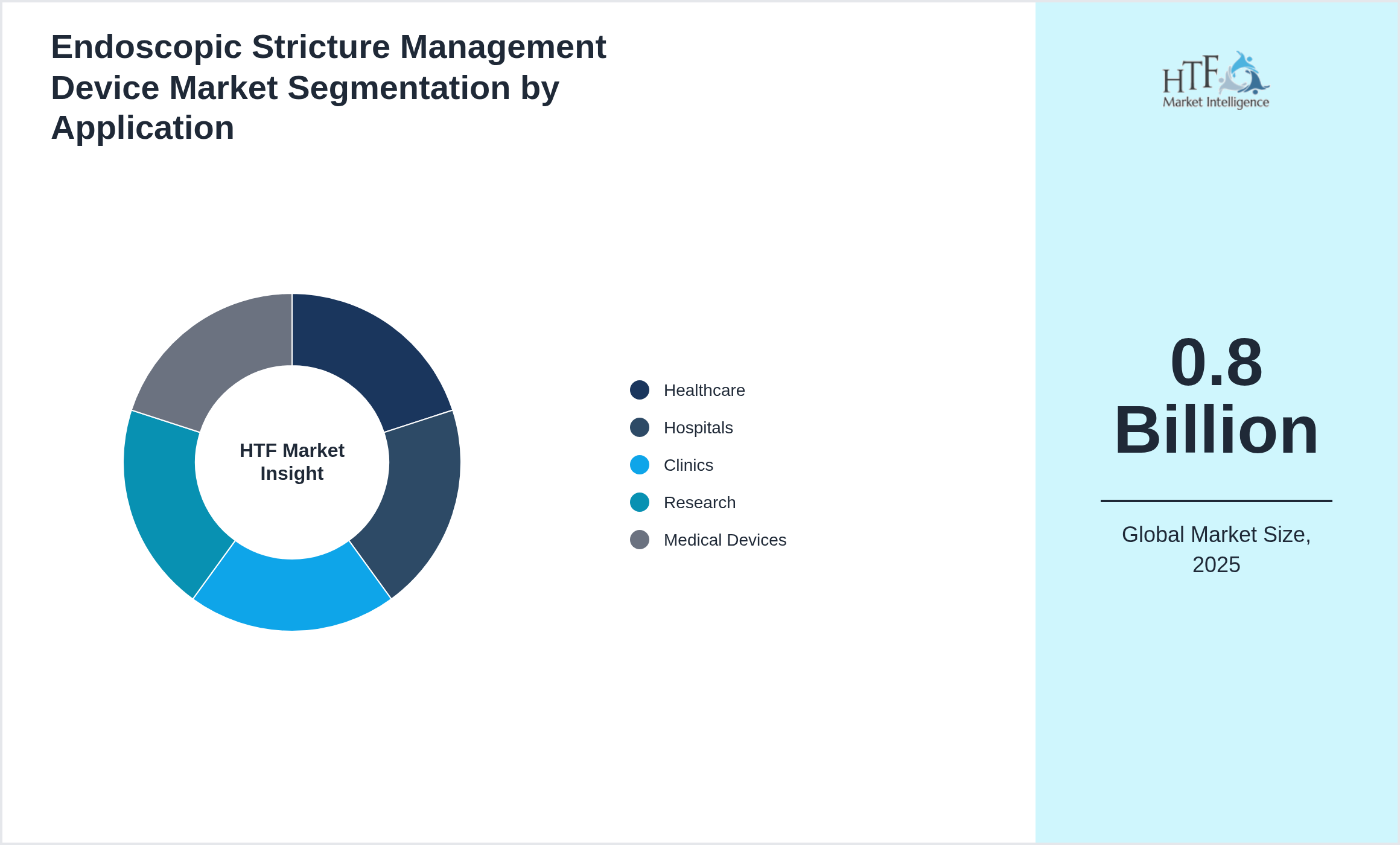
Segmentation by Application
- • Healthcare
- • Hospitals
- • Clinics
- • Research
- • Medical Devices
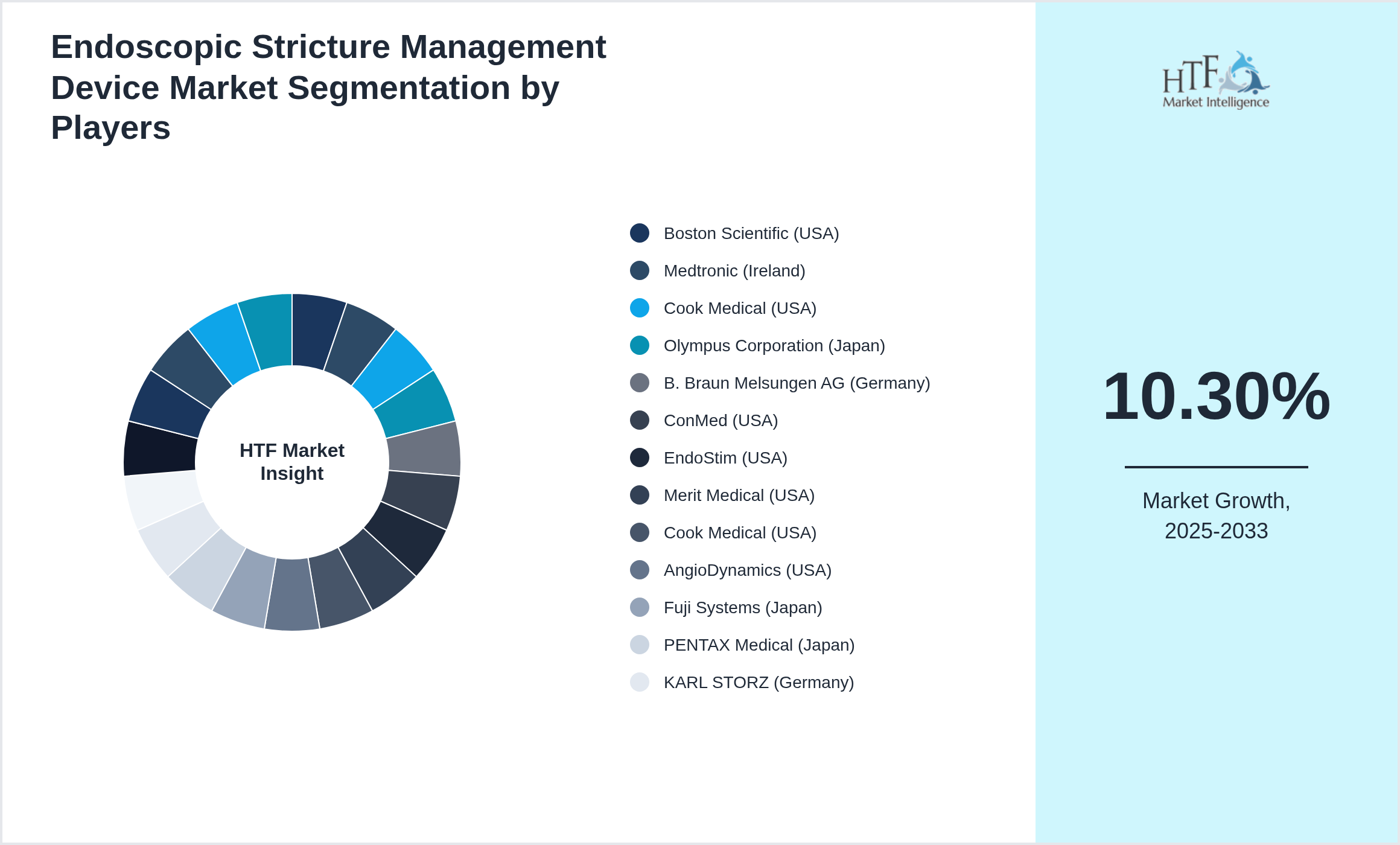
Key Players
Several key players in the Endoscopic Stricture Management Device market is strategically focusing on expanding their operations in developing regions to capture a larger market share, particularly as the year-on-year growth rate for the market stands at N/A. The companies featured in this profile were selected based on insights from primary experts, evaluating their market penetration, product offerings, and geographical reach. By targeting emerging markets, these companies aim to leverage new opportunities, enhance their competitive advantage, and drive revenue growth. This approach not only aligns with their overall business objectives but also positions them to respond effectively to the evolving demands of consumers in these regions.
- • Boston Scientific (USA)
- • Medtronic (Ireland)
- • Cook Medical (USA)
- • Olympus Corporation (Japan)
- • B. Braun Melsungen AG (Germany)
- • ConMed (USA)
- • EndoStim (USA)
- • Merit Medical (USA)
- • Cook Medical (USA)
- • AngioDynamics (USA)
- • Fuji Systems (Japan)
- • PENTAX Medical (Japan)
- • KARL STORZ (Germany)
- • Teleflex (USA)
- • Teleflex Medical (USA)
- • MGB Biopharma (UK)
- • Meril Life Sciences (India)
- • C. R. Bard (USA)
- • Aesculap (Germany)
For the complete companies list, please ask for sample pages.
Market Entropy
Merger & Acquisition
Patent Analysis
Investment and Funding Scenario
Market Estimation Process
Key Highlights
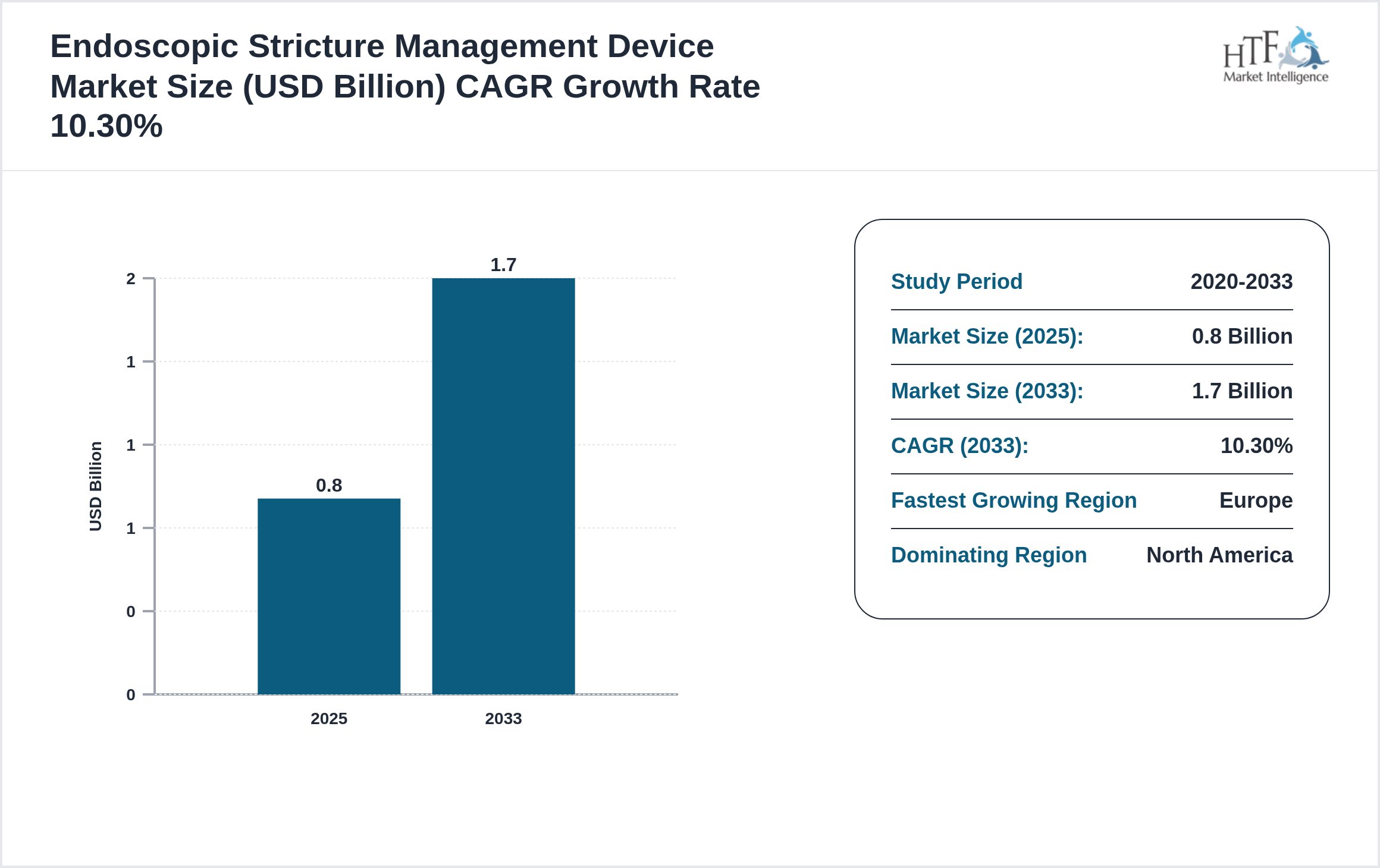
• The Endoscopic Stricture Management Device is growing at a CAGR of 10.30% during the forecasted period of 2025 to 2033
• Year on Year growth for the market is N/A
• North America dominated the market share of 0.8 Billion in 2025
• Based on type, the market is bifurcated into Stent-based Devices, Balloon Dilators, Endoscopic Laser Devices, Electrocautery Devices, Endoscopic Forceps segment, which dominated the market share during the forecasted period
• Based on application, the market is segmented into Application Healthcare, Hospitals, Clinics, Research, Medical Devices is the fastest-growing segment
• Global Import Export in terms of K Tons, K Units, and Metric Tons will be provided if Applicable based on industry best practice
Our Data Collection Process Based on Best Practice
Problem Definition: Clarify research objectives and client needs & identify key questions and market scope.
Data Collection:
Primary Research: Conduct interviews, surveys, and focus groups.
Secondary Research: Analyzed industry reports, market publications, and financial records.
Data Analysis:
Quantitative Analysis: Use statistical tools to identify trends and quantify market size.
Qualitative Analysis: Interpret non-numerical data to understand market drivers and consumer behavior.
Market Segmentation:
Divide the market into distinct segments based on shared characteristics.
Validation and Triangulation:
Cross-verify findings from multiple sources to ensure accuracy and reliability.
Reporting and Recommendations:
Present insights and strategic recommendations in a tailored, actionable report.
Continuous Feedback Loop:
Engage with clients to refine research and ensure alignment with their goals.
Regional Insight
The Endoscopic Stricture Management Device varies widely by region, reflecting diverse economic conditions and consumer preferences. In North America, the focus is on convenience and premium products, driven by high disposable incomes and a strong e-commerce sector. Europe’s market is fragmented, with Western countries emphasizing luxury and organic goods, while Eastern Europe sees rapid growth. Asia-Pacific is a fast-growing region with high demand for high-tech and affordable products, driven by urbanization and rising middle-class incomes. Latin America prioritizes affordability amidst economic fluctuations, with Brazil and Mexico leading in market growth. In the Middle East and Africa, market trends are influenced by cultural preferences, with luxury goods prominent in the Gulf States and gradual growth in sub-Saharan Africa. Global trends like sustainability and digital transformation are impacting all regions.
The North America dominant region currently dominates the market share, fueled by increasing consumption, population growth, and sustained economic progress which collectively enhance market demand. Conversely, the Europe is growing rapidly, driven by significant infrastructure investments, industrial expansion, and rising consumer demand.
- North America
- LATAM
- West Europe
- Central & Eastern Europe
- Northern Europe
- Southern Europe
- East Asia
- Southeast Asia
- South Asia
- Central Asia
- Oceania
- MEA
The Top-Down and Bottom-Up Approaches
The top-down approach begins with a broad theory or hypothesis and breaks it down into specific components for testing. This structured, deductive process involves developing a theory, creating hypotheses, collecting and analyzing data, and drawing conclusions. It is particularly useful when there is substantial theoretical knowledge, but it can be rigid and may overlook new phenomena.
Conversely, the bottom-up approach starts with specific data or observations, from which broader generalizations and theories are developed. This inductive process involves collecting detailed data, analyzing it for patterns, developing hypotheses, formulating theories, and validating them with additional data. While this approach is flexible and encourages the discovery of new phenomena, it can be time-consuming and less structured.
Regulatory Framework
The healthcare sector is overseen by various regulatory bodies that ensure the safety, quality, and efficacy of health services and products. In the United States, the U.S. Department of Health and Human Services (HHS) plays a crucial role in protecting public health and providing essential human services. Within HHS, the Food and Drug Administration (FDA) regulates food, drugs, and medical devices, ensuring they meet safety and efficacy standards. The Centers for Disease Control and Prevention (CDC) focus on disease control and prevention, conducting research, and providing health information to protect public health.
In the United Kingdom, the General Medical Council (GMC) regulates doctors, ensuring they adhere to professional standards. Other important bodies include the General Pharmaceutical Council (GPhC), which oversees pharmacists, and the Nursing and Midwifery Council (NMC), which regulates nurses and midwives. These organizations work to maintain high standards of care and protect patients.
Internationally, the European Medicines Agency (EMA) regulates medicines within the European Union, while the World Health Organization (WHO) provides global leadership on public health issues. Each of these regulatory bodies plays a vital role in ensuring that health care systems operate effectively and safely, ultimately safeguarding public health across different regions.
Report Infographics
| Report Features | Details |
| Base Year | 2025 |
| Based Year Market Size (2025) | 0.8 Billion |
| Historical Period | 2020 to 2025 |
| CAGR (2025 to 2033) | 10.30% |
| Forecast Period | 2025 to 2033 |
| Forecasted Period Market Size ( 2033) | 1.7 Billion |
| Scope of the Report | Stent-based Devices, Balloon Dilators, Endoscopic Laser Devices, Electrocautery Devices, Endoscopic Forceps, Healthcare, Hospitals, Clinics, Research, Medical Devices |
| Regions Covered | North America, LATAM, West Europe, Central & Eastern Europe, Northern Europe, Southern Europe, East Asia, Southeast Asia, South Asia, Central Asia, Oceania, MEA |
| Companies Covered | Boston Scientific (USA), Medtronic (Ireland), Cook Medical (USA), Olympus Corporation (Japan), B. Braun Melsungen AG (Germany), ConMed (USA), EndoStim (USA), Merit Medical (USA), Cook Medical (USA), AngioDynamics (USA), Fuji Systems (Japan), PENTAX Medical (Japan), KARL STORZ (Germany), Teleflex (USA), Teleflex Medical (USA), MGB Biopharma (UK), Meril Life Sciences (India), C. R. Bard (USA), Aesculap (Germany) |
| Customization Scope | 15% Free Customization |
| Delivery Format | PDF and Excel through Email |
Endoscopic Stricture Management Device - Table of Contents
Chapter 1: Market Preface
Chapter 2: Strategic Overview
Chapter 3: Global Endoscopic Stricture Management Device Market Business Environment & Changing Dynamics
Chapter 4: Global Endoscopic Stricture Management Device Industry Factors Assessment
Chapter 5: Endoscopic Stricture Management Device : Competition Benchmarking & Performance Evaluation
Chapter 6: Global Endoscopic Stricture Management Device Market: Company Profiles
Chapter 7: Global Endoscopic Stricture Management Device by Type & Application (2020-2033)
Chapter 8: North America Endoscopic Stricture Management Device Market Breakdown by Country, Type & Application
Chapter 9: Europe Endoscopic Stricture Management Device Market Breakdown by Country, Type & Application
Chapter 10: Asia Pacific Endoscopic Stricture Management Device Market Breakdown by Country, Type & Application
Chapter 11: Latin America Endoscopic Stricture Management Device Market Breakdown by Country, Type & Application
Chapter 12: Middle East & Africa Endoscopic Stricture Management Device Market Breakdown by Country, Type & Application
Chapter 13: Research Finding and Conclusion
Frequently Asked Questions (FAQ):
The Compact Track Loaders market is projected to grow at a CAGR of 6.8% from 2025 to 2030, driven by increasing demand in construction and agricultural sectors.
North America currently leads the market with approximately 45% market share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining regions account for 5% of the global market.
Key growth drivers include increasing construction activities, rising demand for versatile equipment in agriculture, technological advancements in track loader design, and growing preference for compact equipment in urban construction projects.
